
youlike300 เว็บไซต์คาสิโนออนไลน์เว็บตรง ยิ่งเล่นยิ่งรวย
youlike300 เว็บไซต์ที่เราพัฒนาขึ้นเป็นที่นิยมและกระแสร้อนแรงที่สุดในขณะนี้, มีทุกความสนุกที่คุณคาดหวัง. ไม่เพียงเท่านี้, พวกเรายังมีเกมสล็อตออนไลน์ที่กำลังฮิตและได้รับความนิยมมากที่สุดในขณะนี้, ซึ่งถูกคัดสรรมาเพื่อให้สมาชิกและนักเดิมพันทุกระดับได้ทำเงินและทำกำไรอย่างมีประสิทธิภาพที่สุด.
เรามีความมั่นใจว่าท่านทุกท่านจะไม่ผิดหวัง, เนื่องจากเราเปิดโอกาสให้ทุกท่านเข้ามาสร้างรายได้เสริมได้อย่างง่ายๆ และทำเงินง่าย ๆ ที่เราทำเป็น youlike333 ลงทุนน้อยก็ได้กำไรมหาศาล, ยิ่งเล่นยิ่งรวย และที่สำคัญคือ ไม่ต้องกังวลเรื่องขาดทุน, เราได้รับรองว่าท่านจะไม่มีขาดทุนแน่นอน.
สามารถสัมผัสไปกับเกมสล็อตออนไลน์ในรูปแบบใหม่ที่พัฒนาขึ้นอย่างต่อเนื่อง คาสิโนออนไลน์1688 ซึ่งถูกออกแบบมาเพื่อให้สมาชิกนักเดิมพันทุกระดับได้ลิ้มลองความตื่นเต้น สนุกสนานและเร้าใจไปกับการทำเงิน คุณสามารถเล่นได้ทุกที่ทุกเวลา

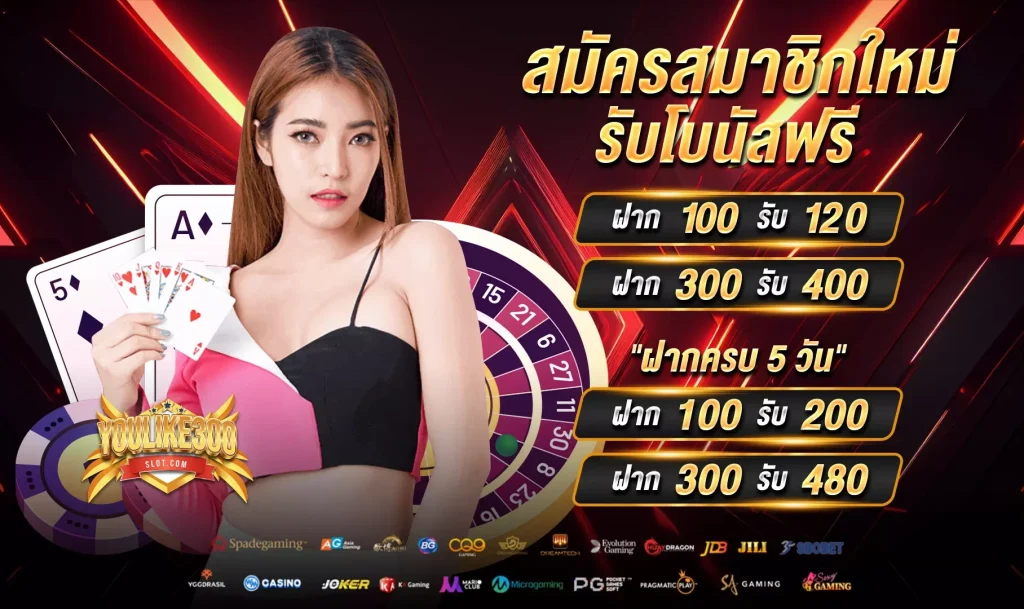
เว็บไซต์สล็อตที่มีค่ายสล็อตรองรับมากที่สุด
ที่นี่คุณจะได้สนุกกับเกมสล็อตบนเว็บไซต์ที่มีเกมเบทที่ถูกใจและรองรับระบบโอนเงินผ่าน True Wallet ที่เราพร้อมเป็นศูนย์กลางทางด้านการบริการ เกมสล็อต ชั้นนำของประเทศไทย คาสิโนออนไลน์ เว็บตรง มีเกมสล็อตมากมายที่คุณสามารถเลือกเล่น, ทุกเกมล้วนแต่ได้รับมาตรฐานสากล, ทำให้คุณสามารถทำเงินและทำกำไรได้จริง ท่านสามารถทำรายการทั้งฝากและถอนเงินผ่าน True Wallet ซึ่งถือว่าเป็นช่องทางที่นิยมมาก, เพราะการเติมเงินเครดิตนั้นทำได้ง่ายมากกว่าผ่านธนาคาร, และที่สำคัญคือไม่มีค่าธรรมเนียม. เรายังมีระบบที่เสถียรทันสมัย, ไม่ล่มบ่อยเหมือนธนาคารทั่วไป และไม่มีปิดทำการโอนเงิน.
ไม่เพียงเท่านี้ คาสิโนออนไลน์ fafa789 ยังให้บริการที่ไม่เปิดทำการลงทุนในช่วงเวลากลางคืน, รับรองว่าคุณจะไม่เจอปัญหาความล่าช้าในการเติมเงินหรือปัญหาเหตุขัดข้องในระหว่างการเติมเงิน. เพื่อความสะดวกสบาย, เราได้พัฒนาระบบการเติมผ่านช่องทาง True Wallet ที่ชื่อ youlike191 ขึ้นมาเพื่อทำให้รายการง่ายรวดเร็ว และทันใจ, โอนง่าย, และถอนไวภายใน 3 นาที. ทีมงานของเราพร้อมให้บริการทุกเวลา เพื่อให้คุณได้ประสบการณ์การเล่นเกมที่ดีที่สุด

youlike300 ปลอดภัยและมั่นคง
คาสิโนออนไลน์1688 มุ่งเน้นทำให้ชีวิตของท่านสะดวกสบายมากขึ้นอย่างแน่นอน. เมื่อท่านทดลองใช้บริการของ เว็บสล็อต ตรง ท่านจะพบว่าไม่ต้องกังวลเรื่องการเงิน. การฝากเงินและการถอนเงินบนเว็บของเรามีความปลอดภัยสูงสุดและมีความมั่นคง. ระบบธุรกรรมการเงินทำงานโดยอัตโนมัติ, ทำให้ท่านสามารถลงเดิมพันได้ทันที
youlike300 ยังมีช่องทางเข้าโดยตรงที่นำเสนอบริการเกมพนันออนไลน์แบบครบถ้วนที่สุด. ไม่ต้องไปหาเล่นจากที่ไหนอีก, เพราะที่นี่คุณจะพบทุกรูปแบบของเกมในเว็บเดียว. เรามีเกมสล็อต, คาสิโนสด, แทงบอล, และแทงหวยให้บริการครบจบ. ท่านสามารถลุ้นรับโบนัสและแจ็กพอตได้อย่างอิสระ. เว็บของเราเป็นเว็บตรงที่ไม่ผ่านเอเยนต์, และมีการอัปเดตระบบอยู่ตลอดเวลา. ท่านจะได้สัมผัสประสบการณ์การเล่นเกมที่ดีที่สุด
ทางเรามีโปรโมชั่นที่น่าตื่นเต้นและสิทธิพิเศษมากมาย
สำหรับท่านที่ต้องการสนุกและสร้างรายได้จากเกมสล็อตออนไลน์, วันนี้เป็นโอกาสที่ดีที่จะเข้าร่วมกับเว็บไซต์ของเรา! youlike333 มีโปรโมชั่นที่คุ้มค่ารอให้ท่านได้รับ, และทุกคนที่สมัครเป็นสมาชิกจะได้รับสิทธิพิเศษโดยไม่มีกั๊ก. เริ่มต้นสร้างรายได้ด้วยการเล่นสล็อตออนไลน์ผ่านเว็บไซต์ของเราง่ายมากเพียงใด!
กระบวนการสมัครสมาชิกเป็นเรื่องที่ง่ายมาก เพียงแค่เข้าสู่หน้าเว็บไซต์หลักและคลิกที่เมนู “สมัครสมาชิก”. หลังจากนั้น, กรอกรายละเอียดส่วนตัวของท่านและส่งข้อมูลเข้าสู่ระบบ ทุกสิ่งนี้จะถูกบันทึกโดยอัตโนมัติ. ระบบจะทำการส่ง SMS เพื่อยืนยันตัวตนและรหัส otp ให้ท่าน, จากนั้นคุณสามารถล็อกอินเข้าสู่ระบบ คาสิโนออนไลน์ 1688 ครั้งแรกได้ทันที
ท่านจะได้เข้าสู่โลกของเกมสล็อตที่ดีที่สุดของเมืองไทย. ทางเรายังมีโปรโมชั่นที่น่าตื่นเต้นและสิทธิพิเศษมากมาย ไม่ว่าจะเป็นโปรสมัครครั้งแรก, เติมเงินครั้งแรก, หรือกิจกรรมรายสัปดาห์, ท่านสามารถรับโปรโมชั่นเหล่านี้ได้โดยไม่ต้องลุ้นให้เสียเวลา ท่านจะได้สนุกไปพร้อมกับการกดสล็อตและสัมผัสบรรยากาศของการเล่นเกมที่น่าตื่นเต้น

youlike300 เข้าเล่นง่ายไม่ยุ่งยาก ไม่มีขั้นต่ำ
เล่น เว็บสล็อต ตรง slot ไม่ยุ่งยากและสามารถทำเงินได้อย่างรวดเร็ว โดยไม่ต้องมีขั้นตอนที่ซับซ้อนหรือซับซ้อนในการคำนวณ. คุณเพียงแค่หมุนวงล้อและสามารถเสี่ยงโชคได้อย่างรวดเร็ว คาสิโนออนไลน์ 1688 ท่านจะได้รับเงินรางวัลอย่างมหาศาลและมีสิทธิ์ในการลุ้นรับอัตราการจ่ายเงินรางวัลที่สวยงาม. แจ็กพอตก็มีโอกาสแตกออกมาเมื่อใดก็ตาม และท่านสามารถลงทุนเพียงไม่กี่บาทเท่านั้น ซึ่งมีความคุ้มค่ามากที่สุด.
อีกทั้ง, การลงทุนใน youlike300 slot นั้นไม่มีขั้นต่ำ ท่านสามารถเริ่มต้นที่ราคาเพียง 1 บาทเท่านั้น ทำให้ท่านสามารถพลิกชีวิตของคุณให้ดียิ่งขึ้นได้โดยไม่ต้องเสี่ยงเงินมาก ทางเว็บไซต์ของเราได้รวบรวมเกมสล็อตจากค่ายเกมชั้นนำทั่วโลกเพื่อให้ท่านได้เลือกเล่นอย่างเต็มที่. เราอัปเดตเกมสล็อตใหม่ๆ เพื่อให้คุณได้มีการเลือกมากมายโดยไม่มีขีดจำกัด. เพื่อนๆ ที่ชื่นชอบการเล่นเกมและต้องการมีรายได้เสริม, เชิญเข้าร่วมเล่นเกมสล็อตที่นี่เพื่อสนุกและได้รับเงินรางวัลอย่างมหาศาล

ฝาก-ถอน รวดเร็วและปลอดภัย สามารถทำได้ตลอด 24 ชั่วโมง
การทำธุรกรรมทางการเงินที่รวดเร็วและมีความปลอดภัยมีบทบาทสำคัญในการเลือก คาสิโนออนไลน์ เว็บตรง ด้วยระบบฝากถอนอัตโนมัติที่สามารถทำได้ตลอด 24 ชั่วโมง, ท่านจะมีความมั่นใจในการทำธุรกรรมที่รวดเร็วและมีประสิทธิภาพ นอกจากนี้ ไม่มีข้อกำหนดขั้นต่ำทำให้เป็นทางเลือกที่เหมาะสมสำหรับนักพนันทุกระดับ
เว็บสล็อต คาสิโนออนไลน์ fafa789 ที่รองรับ True Wallet ทำให้ท่านสามารถเพลิดเพลินไปกับความสนุกและโชคลาภที่เกี่ยวข้องกับการเล่นสล็อตออนไลน์ได้อย่างสมบูรณ์แบบ. คุณภาพที่ปลอดภัยและสะดวกสบายของ True Wallet ทำให้มีการทำธุรกรรมที่ง่ายและมีประสิทธิภาพ, เป็นทางเลือกที่เหมาะสมสำหรับนักพนันทุกระดับ




